A neutron is a neutral particle belonging to the class of hadrons. Discovered in 1932 by the English physicist J. Chadwick. Together with protons, neutrons are part of atomic nuclei. The electric charge of the neutron is zero. This is confirmed by direct measurements of the charge by the deflection of the neutron beam in strong electric fields, which showed that (here is the elementary electric charge, that is, the absolute value of the electron charge). Indirect data provide an estimate. The spin of the neutron is 1/2. As a hadron with half-integer spin, it belongs to the group of baryons (see Proton). Every baryon has an antiparticle; the antineutron was discovered in 1956 in experiments on the scattering of antiprotons by nuclei. The antineutron differs from the neutron in the sign of the baryon charge; a neutron, like a proton, has a baryon charge.
Like a proton and other hadrons, a neutron is not a truly elementary particle: it consists of one m-quark with an electric charge and two -quarks with a charge, connected by a gluon field (see. Elementary particles, Quarks, Strong interactions).
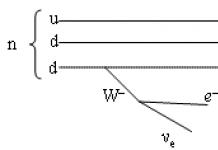
Neutrons are stable only in the composition of stable atomic nuclei. A free neutron is an unstable particle that decays into a proton, an electron and an electron antineutrino (see Beta decay):. The neutron lifetime is s, that is, about 15 minutes. In matter in a free form, neutrons exist even less due to their strong absorption by nuclei. Therefore, they arise in nature or are obtained in the laboratory only as a result of nuclear reactions.
The energy balance of various nuclear reactions was used to determine the difference between the masses of the neutron and proton: MeV. By comparing it with the proton mass, we obtain the neutron mass: MeV; this corresponds to r, or, where is the electron mass.
The neutron participates in all kinds of fundamental interactions (see. The unity of the forces of nature). Strong interactions bind neutrons and protons in atomic nuclei. An example of a weak interaction - beta decay of a neutron - has already been considered here. Does this neutral particle participate in electromagnetic interactions? The neutron has an internal structure, and in it, with general neutrality, there are electric currents, leading, in particular, to the appearance of a magnetic moment in the neutron. In other words, in a magnetic field, a neutron behaves like a compass needle.
This is just one example of its electromagnetic interaction.
The search for the electric dipole moment of the neutron, for which the upper bound was obtained:. Here, scientists from the Leningrad Institute of Nuclear Physics of the Academy of Sciences of the USSR succeeded in staging the most effective experiments. Searches for the dipole moment of neutrons are important for understanding the mechanisms of violation of invariance with respect to time reversal in microprocesses (see Parity).
Gravitational interactions of neutrons were observed directly from their fall in the Earth's gravitational field.
A conditional classification of neutrons by their kinetic energy has now been adopted: slow neutrons eV, there are many varieties of them), fast neutrons (eV), high-energy eV). Very slow neutrons (eV), called ultracold, have very interesting properties. It turned out that ultracold neutrons can accumulate in "magnetic traps" and even orient their spins there in a certain direction. With the help of magnetic fields of a special configuration, ultracold neutrons are isolated from the absorbing walls and can "live" in a trap until they decay. This allows many subtle experiments to be carried out to study the properties of neutrons.
Another method of storing ultracold neutrons is based on their wave properties. At low energies, the de Broglie wavelength (see Quantum Mechanics) is so great that neutrons are reflected from the nuclei of matter in the same way that light is reflected from a mirror. Such neutrons can simply be stored in a closed "bank". This idea was expressed by the Soviet physicist Ya. B. Zel'dovich in the late 1950s, and the first results were obtained in Dubna, at the Joint Institute for Nuclear Research, almost a decade later. Recently, Soviet scientists managed to build a vessel in which ultracold neutrons live until they naturally decay.
Free neutrons are able to actively interact with atomic nuclei, causing nuclear reactions. As a result of the interaction of slow neutrons with matter, resonance effects, diffraction scattering in crystals, etc. can be observed. Due to these features, neutrons are widely used in nuclear and solid state physics. They play an important role in nuclear power, in the production of transuranium elements and radioactive isotopes, and find practical application in chemical analysis and geological exploration.
NEUTRON
Neutron
Neutron- a neutral particle belonging to the class of baryons. Together with the proton, the neutron forms atomic nuclei. The neutron mass is mn = 938.57 MeV / s 2 ≈ 1.675 · 10 -24 g. The neutron, like a proton, has a spin of 1 / 2ћ and is a fermion .. It also has a magnetic moment μ n = - 1.91μ N, where μ N = е ћ / 2m рс - nuclear magneton (m р - proton mass, Gaussian system of units is used). The size of a neutron is about 10 -13 cm. It consists of three quarks: one u-quark and two d-quarks, i.e. its quark structure is udd.
A neutron, being a baryon, has a baryon number B = +1. A neutron is unstable in a free state. Since it is somewhat heavier than a proton (by 0.14%), it decays with the formation of a proton in the final state. In this case, the law of conservation of the baryon number is not violated, since the baryon number of the proton is also +1. As a result of this decay, an electron e - and an electron antineutrino e are also formed. Decay occurs due to weak interaction.

Decay scheme n → p + e - + e.
The lifetime of a free neutron is τ n ≈ 890 sec. In an atomic nucleus, a neutron can be as stable as a proton.
The neutron, being a hadron, participates in a strong interaction.
The neutron was discovered in 1932 by J. Chadwick.
What is a neutron? This question most often arises among people who are not engaged in nuclear physics, because under the neutron is understood in it an elementary particle that does not have an electric charge and has a mass that is 1838.4 times greater than the electronic one. Together with the proton, whose mass is slightly less than the mass of the neutron, it is the "building block" of the atomic nucleus. In elementary particle physics, a neutron and a proton are assumed to be two different forms of one particle - a nucleon.
A neutron is present in the composition of the nuclei of atoms for each chemical element, the only exception is the hydrogen atom, the nucleus of which is one proton. What is a neutron, what structure does it have? Although it is called an elementary "brick" of the nucleus, it still has its own internal structure. In particular, it belongs to the baryon family and consists of three quarks, two of which are down-type quarks, and one is up-type. All quarks have a fractional electric charge: the upper one is positively charged (+2/3 of the electron charge), and the lower one is negative (-1/3 of the electron charge). That is why the neutron has no electric charge, because it is simply compensated for by its constituent quarks. However, the magnetic moment of the neutron is not zero.
In the composition of the neutron, the definition of which was given above, each quark is connected to the rest with the help of a gluon field. The gluon is the particle responsible for the formation of nuclear forces.
In addition to the mass in kilograms and atomic mass units, in nuclear physics the mass of a particle is also described in GeV (gigaelectronvolts). This became possible after Einstein discovered his famous equation E = mc 2, which relates energy to mass. What is a Neutron in GeV? This is a value of 0.0009396, which is slightly higher than that of the proton (0.0009383).
Stability of the neutron and atomic nuclei

The presence of neutrons in atomic nuclei is very important for their stability and the possibility of the existence of the atomic structure itself and matter as a whole. The fact is that the protons, which also make up the atomic nucleus, have a positive charge. And their rapprochement at close distances requires the expenditure of huge energies due to the Coulomb electrical repulsion. The nuclear forces acting between neutrons and protons are 2-3 orders of magnitude stronger than the Coulomb forces. Therefore, they are able to hold positively charged particles at close distances. Nuclear interactions are short-range and manifest themselves only within the size of the nucleus.
The formula for neutrons is used to find their amount in the nucleus. It looks like this: number of neutrons = atomic mass of an element - atomic number in the periodic table.
A free neutron is an unstable particle. Its average lifetime is 15 minutes, after which it disintegrates into three particles:
- electron;
- proton;
- antineutrino.
Prerequisites for the discovery of the neutron
The theoretical existence of the neutron in physics was proposed back in 1920 by Ernest Rutherford, who thus tried to explain why atomic nuclei do not fall apart due to the electromagnetic repulsion of protons.
Even earlier, in 1909 in Germany, Bothe and Becker established that if light elements, such as beryllium, boron or lithium, are irradiated with high-energy alpha particles from polonium, then radiation is formed that passes through any thickness of various materials. They assumed that this radiation was gamma, but no such radiation known at that time had such a high penetrating power. Bothe and Becker's experiments were not properly interpreted.
Discovery of the neutron

The existence of the neutron was discovered by the English physicist James Chadwick in 1932. He studied the radioactive radiation of beryllium, conducted a series of experiments, obtaining results that did not coincide with those predicted by the physical formulas: the energy of the radioactive radiation was much higher than the theoretical values, and the law of conservation of momentum was also violated. Therefore, it was necessary to accept one of the hypotheses:
- Or the angular momentum is not conserved during nuclear processes.
- Either the radioactive radiation is made up of particles.
The scientist rejected the first assumption, since it contradicts fundamental physical laws, therefore he accepted the second hypothesis. Chadwick showed that radiation in his experiments is formed by particles with zero charge, which have a strong penetrating ability. In addition, he was able to measure the mass of these particles, finding that it is slightly larger than that of a proton.
Slow and fast neutrons
Depending on the energy possessed by a neutron, it is called slow (about 0.01 MeV) or fast (about 1 MeV). This classification is important because some of its properties depend on the speed of the neutron. In particular, fast neutrons are well captured by nuclei, leading to the formation of their isotopes, and causing their fission. Slow neutrons are poorly captured by the nuclei of almost all materials, so they can freely pass through thick layers of matter.
Role of the neutron in the fission of a uranium nucleus

If you ask yourself what a neutron is in nuclear power, then we can say with confidence that this is a means of inducing the process of fission of a uranium nucleus, accompanied by the release of high energy. During this fission reaction, neutrons of various speeds are also generated. In turn, the generated neutrons induce the decay of other uranium nuclei, and the reaction proceeds in a chain manner.

If the uranium fission reaction is uncontrolled, it will lead to an explosion of the reaction volume. This effect is used in nuclear bombs. The controlled fission reaction of uranium is the source of energy in nuclear power plants.
What is a neutron in physics. Its strony, as well as an important role in the stability of the atomic nucleus. The history of the discovery of the neutron. Properties of Fast and Slow Neutron ...
What is a neutron in physics: structure, properties and use
From Masterweb
31.05.2018 12:00What is a neutron? This question most often arises among people who are not engaged in nuclear physics, because under the neutron is understood in it an elementary particle that does not have an electric charge and has a mass that is 1838.4 times greater than the electronic one. Together with the proton, whose mass is slightly less than the mass of the neutron, it is the "building block" of the atomic nucleus. In elementary particle physics, a neutron and a proton are assumed to be two different forms of one particle - a nucleon.
The structure of the neutron
A neutron is present in the composition of the nuclei of atoms for each chemical element, the only exception is the hydrogen atom, the nucleus of which is one proton. What is a neutron, what structure does it have? Although it is called an elementary "brick" of the nucleus, it still has its own internal structure. In particular, it belongs to the baryon family and consists of three quarks, two of which are down-type quarks, and one is up-type. All quarks have a fractional electric charge: the upper one is positively charged (+2/3 of the electron charge), and the lower one is negative (-1/3 of the electron charge). That is why the neutron has no electric charge, because it is simply compensated for by its constituent quarks. However, the magnetic moment of the neutron is not zero.
In the composition of the neutron, the definition of which was given above, each quark is connected to the rest with the help of a gluon field. The gluon is the particle responsible for the formation of nuclear forces.
In addition to the mass in kilograms and atomic mass units, in nuclear physics the mass of a particle is also described in GeV (gigaelectronvolts). This became possible after Einstein's discovery of his famous equation E = mc2, which relates energy to mass. What is a Neutron in GeV? This is a value of 0.0009396, which is slightly higher than that of the proton (0.0009383).
Stability of the neutron and atomic nuclei

The presence of neutrons in atomic nuclei is very important for their stability and the possibility of the existence of the atomic structure itself and matter as a whole. The fact is that the protons, which also make up the atomic nucleus, have a positive charge. And their rapprochement at close distances requires the expenditure of huge energies due to the Coulomb electrical repulsion. The nuclear forces acting between neutrons and protons are 2-3 orders of magnitude stronger than the Coulomb forces. Therefore, they are able to hold positively charged particles at close distances. Nuclear interactions are short-range and manifest themselves only within the size of the nucleus.
The formula for neutrons is used to find their amount in the nucleus. It looks like this: number of neutrons = atomic mass of an element - atomic number in the periodic table.
A free neutron is an unstable particle. Its average lifetime is 15 minutes, after which it disintegrates into three particles:
- electron;
- proton;
- antineutrino.
Prerequisites for the discovery of the neutron
The theoretical existence of the neutron in physics was proposed back in 1920 by Ernest Rutherford, who thus tried to explain why atomic nuclei do not fall apart due to the electromagnetic repulsion of protons.
Even earlier, in 1909 in Germany, Bothe and Becker established that if light elements, such as beryllium, boron or lithium, are irradiated with high-energy alpha particles from polonium, then radiation is formed that passes through any thickness of various materials. They assumed that this radiation was gamma, but no such radiation known at that time had such a high penetrating power. Bothe and Becker's experiments were not properly interpreted.
Discovery of the neutron

The existence of the neutron was discovered by the English physicist James Chadwick in 1932. He studied the radioactive radiation of beryllium, conducted a series of experiments, obtaining results that did not coincide with those predicted by the physical formulas: the energy of the radioactive radiation was much higher than the theoretical values, and the law of conservation of momentum was also violated. Therefore, it was necessary to accept one of the hypotheses:
- Or the angular momentum is not conserved during nuclear processes.
- Either the radioactive radiation is made up of particles.
The scientist rejected the first assumption, since it contradicts fundamental physical laws, therefore he accepted the second hypothesis. Chadwick showed that radiation in his experiments is formed by particles with zero charge, which have a strong penetrating ability. In addition, he was able to measure the mass of these particles, finding that it is slightly larger than that of a proton.
Slow and fast neutrons
Depending on the energy possessed by a neutron, it is called slow (about 0.01 MeV) or fast (about 1 MeV). This classification is important because some of its properties depend on the speed of the neutron. In particular, fast neutrons are well captured by nuclei, leading to the formation of their isotopes, and causing their fission. Slow neutrons are poorly captured by the nuclei of almost all materials, so they can freely pass through thick layers of matter.
Role of the neutron in the fission of a uranium nucleus

If you ask yourself what a neutron is in nuclear power, then we can say with confidence that this is a means of inducing the process of fission of a uranium nucleus, accompanied by the release of high energy. During this fission reaction, neutrons of various speeds are also generated. In turn, the generated neutrons induce the decay of other uranium nuclei, and the reaction proceeds in a chain manner.

If the uranium fission reaction is uncontrolled, it will lead to an explosion of the reaction volume. This effect is used in nuclear bombs. The controlled fission reaction of uranium is the source of energy in nuclear power plants.
Kievyan street, 16 0016 Armenia, Yerevan +374 11 233 255